
Tap to Read ➤
What Does STP Mean in the Context of Chemistry
Prachi Patkar

STP - short for Standard Temperature and Pressure, is a standard which is established for data comparison. Read in this story about STP standard, its definition, and importance.

Standards
Earlier, the standard pressure and temperature were defined as 101.325 kPa and 15 °C.
Earlier, the standard pressure and temperature were defined as 101.325 kPa and 15 °C.
Everything that we come across or use in our daily lives uses a certain standard of measurement. Price can be expressed in terms of dollars, weight can be expressed in terms of pounds and so on. Consider a parameter 'length'.

What would happen if length had no unit to define its magnitude. How would we measure the lengths? Hence, standards such as centimeter, millimeter, kilometer, and so on were defined.

Matter can be solid, liquid, or gaseous. When the temperature or pressure of a substance changes, its properties such as boiling point, velocity, viscosity change. In case we wish to compare between two or more things in terms of these properties, we need a set standard of measuring units. This is called STP. They find wide applications in the branch of chemistry called thermodynamics.
STP also called 'Standard Temperature and Pressure' is considered to be a value of 0o Celsius for temperature and 1 bar for pressure. Gases expand and contract depending on their temperature and pressure. Hence, a standard unit helps understand the degree of volume change in these gases.

The standard values of temperature and pressure by IUPAC (International Union of Pure and Applied Chemistry) are currently set as follows:

| Standard Temperature value: 0 oC/ 273.15 o K/32oF Standard Pressure Value: 1 bar/0.987 atm/ 100 KPa |
The atmospheric pressure at the sea level is considered as the standard value of pressure, whereas the freezing point of water at the same pressure is considered to be the standard temperature.

Units of STP
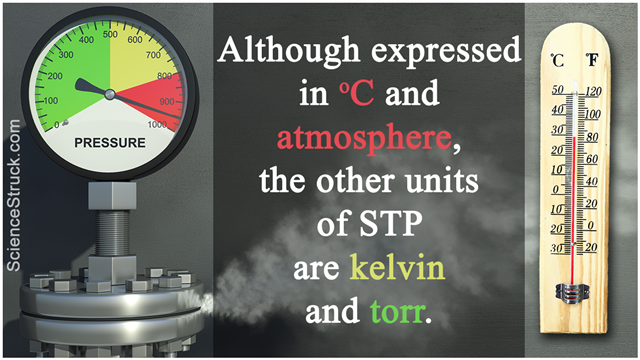
Although expressed in oC and atmosphere, the other units of STP are kelvin and torr.
In chemistry, when we deal with mass flow applications, we come across many volumetric units, hence, a standard is very essential for reference.

Problem I: Given the standard temperature and pressure, find the volume occupied by an ideal gas.
Solution: Ideal gas law equation:
Solution: Ideal gas law equation:

| PV = nRT |

Where P is the standard pressure, T is the standard temperature, n is the number of moles which is 1, R = 8.31441 J K-1 mol-1, and V is the molar volume. Thus, substituting given values in the equation to find the volume.

V = nRT/P = (1 mol)(0.08206 L atm)(273)/1 = 22.4 L
Hence, proven that 1 mole of an ideal gas requires 22.414 L space.

Advantages of STP
1) Makes comparisons between sets of data simpler.
2) Simplifies calculations as the quantities to be compared will have the same units.
2) Simplifies calculations as the quantities to be compared will have the same units.

Think about what would happen if our clothes came with no sizes assigned. There would be a whole lot of confusion while choosing clothes. To sum it all up, everything zeroes down to set standards.

Consistency should be maintained when it comes to calculations and measurements, it just makes comparisons simpler. Hence, standards are used worldwide by everyone - right from navigators, scientists, physicists, pilots, chemists to common man.